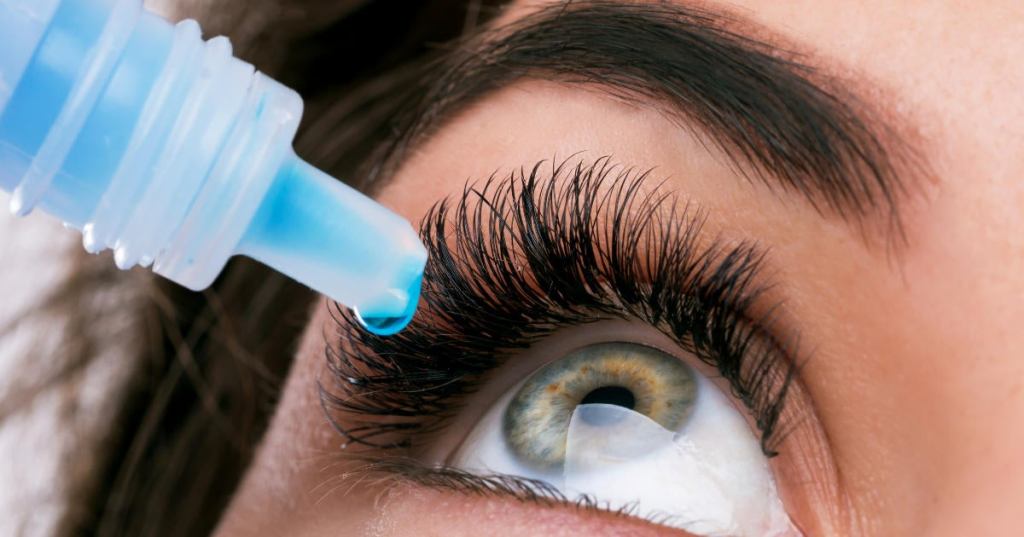
In response to an outbreak of “extensively drug-resistant” bacteria across the U.S., the Food and Drug Administration has moved to restrict imports of eye drops made by an Indian healthcare firm. A recall has been issued for artificial tears produced by Global Pharma Healthcare Private Limited under the brand names EzriCare and Delsam Pharma. According to the FDA, the company failed to comply with several manufacturing regulations, such as “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging. “FDA is warning consumers and health care practitioners not to purchase and immediately stop using EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears due to potential bacterial contamination. Using contaminated artificial tears increases risk of eye infections that could result in blindness or death,” the agency said in a statement on Feb. 2. The regulatory agency’s warning comes after the Centers for Disease Control and Prevention alerted doctors across the country on Tuesday about an unprecedented outbreak of Pseudomonas aeruginosa that had sickened at least 55 people over a dozen states, reported CBS News.
The outbreak strain named Verona Integron-mediated Metallo-β-lactamase (VIM) and Guiana-Extended Spectrum-β-Lactamase (GES)-producing carbapenem-resistant (VIM-GES-CRPA) is so rare that it has never been observed before in the U.S. However, EzriCare eye drops opened by patients in two states were found to contain strains of bacteria similar to those associated with the outbreak. “VIM-GES-CRPA recovered from opened bottles could represent either bacterial contamination during use or during the manufacturing process. Testing of unopened bottles of EzriCare Artificial Tears is ongoing,” the CDC said. At least one person has died following a “systemic infection” resulting from the bacteria. So far, at least five of the 11 patients who have had infections directly in their eyes have lost their vision, a CDC spokesperson said. Patients in the outbreak have developed infections in various parts of their bodies, including respiratory and urinary tract infections. Doctors find treating this strain particularly challenging since it is resistant to at least a dozen different antibiotics tested by the CDC, except for cefiderocol. “Patients who have used EzriCare preservative-free artificial tears and who have signs or symptoms of an eye infection should seek medical care immediately,” the agency said.
Videos by PopCulture.com
Redness in the eye, increased sensitivity to light, and blurry vision can also be signs of an infection. The symptoms can include pain or discomfort in the eye and yellow, green, or clear discharge releasing from it. The eye drops made up part of one of Amazon‘s top ten top-selling products last year regarding “dry eye relief.” Yet, by January, the FDA added Global Pharma to its infamous “red list” of banned imports for failing to comply with manufacturing standards and not adequately responding to its records request. FDA said the company violated its regulations for producing and selling its eye drops in multiuse bottles without preservatives, a practice that can, between uses, lead to bacteria growth. According to Global Pharma, it is “fully cooperating” with federal authorities. “Thus far we have not determined whether our manufacturing facility is the source of the contamination. Nevertheless, out of an abundance of caution, we are recalling the products at issue,” the company said.